NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-04
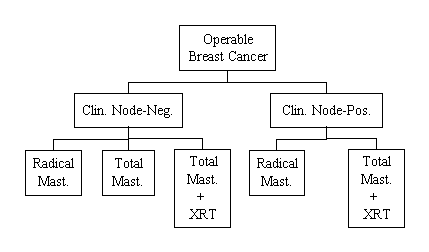
A Protocol for the Evaluation of Radical Mastectomy and Total Mastectomy With and Without Radiation in the Primary Treatment of Cancer of the Female Breast
Specific Aims
To determine in patients with clinically negative axillary nodes whether: (1) total mastectomy (TM) followed by axillary dissection for those patients who subsequently develop positive nodes is as effective a therapy as is radical mastectomy (RM), and (2) total mastectomy with postoperative regional radiation (TMR) is as effective a treatment as RM or TM with postponement of axillary dissection until positive nodes occur. The primary aim in patients with clinically positive nodes is to ascertain whether RM and TMR are equivalent procedures. In addition to supplying information relative to the merits of the various treatments, this investigation also provides data of biological significance, particularly that which confirms or repudiates the worth of en bloc dissection in cancer surgery.