NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-09
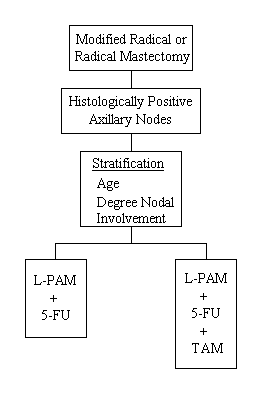
A Protocol to Compare Combined Chemotherapy With and Without Tamoxifen in the Management of Patients With Surgically Curable Breast Cancer
Specific Aims
To determine (1) whether L-PAM + 5-FU + tamoxifen (PFT) with surgery is more effective than surgery with L-PAM + 5-FU (PF) and (2) whether tamoxifen in combination with L-PAM + 5-FU (PFT) is more effective in patients whose tumors had positive estrogen-receptor sites.