NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-14
A Clinical Trial to Assess Tamoxifen in Patients With Primary Breast Cancer and Negative Axillary Nodes Whose Tumors are Positive for Estrogen Receptors
Specific Aims
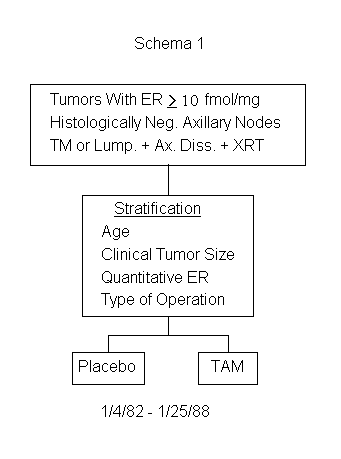
To determine whether tamoxifen is more effective than no treatment with respect to disease-free survival and survival in patients with negative axillary nodes and estrogen-receptor-positive tumors. (See Schema 1)
In conjunction with Protocol B-13, this protocol will also permit evaluation of treatment failure and survival in untreated patients relative to their hormone-receptor levels.
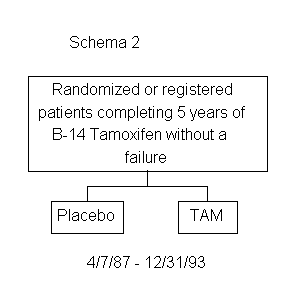
Protocol B-14 was originally designed to treat patients for a total of 2 years but was subsequently modified to maintain treatment through 5 years. In 1987, an extension of B-14 was initiated to evaluate the effectiveness of tamoxifen for 10 years; specifically, to determine whether an additional 5 years of tamoxifen therapy is effective in prolonging disease-free survival and survival in patients with negative nodes and estrogen-receptor-positive tumors. Patients who complete the initially assigned 5 years of tamoxifen therapy are rerandomized to 5 additional years of either tamoxifen or placebo. (See Schema 2)
Randomization for the original protocol objectives was terminated January 25, 1988. From that date until October 17, 1988, all eligible patients were registered to tamoxifen in an effort to enhance the pool of patients eligible for rerandomization at 5 years. At that time protocols B-19 and B-20 opened.