NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
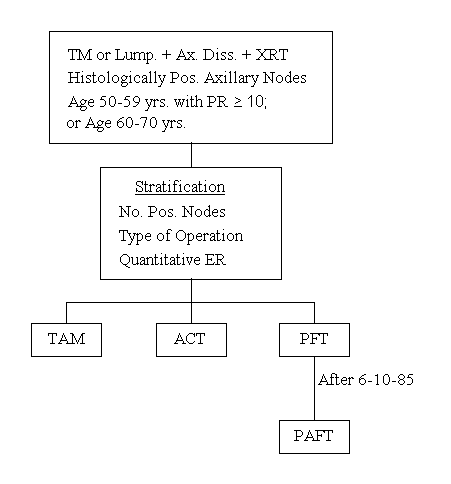
Protocol B-16
A Three-Arm Clinical Trial Comparing Tamoxifen Alone with L-PAM, 5-FU, Adriamycin and Tamoxifen or with Short, Intensive Adriamycin-Cyclophosphamide and Tamoxifen in Positive-Node Patients Having the Following Age and Receptor Criteria:
50-59 Years - PR = 10 fmol or more, regardless of ER
60-70 Years - All Patients
Specific Aims
To determine, in patients with histologically positive axillary nodes who are classified as tamoxifen-responsive, whether tamoxifen alone is as effective as (1) PAF + tamoxifen, or (2) short-course, intensive chemotherapy with AC + tamoxifen, with respect to disease-free survival and survival. The efficacy of ACT relative to PAFT will also be evaluated.