NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-25
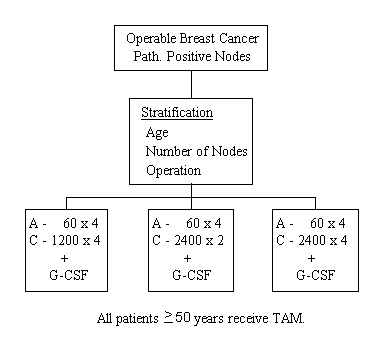
A Clinical Trial to Evaluate the Effect of Dose Intensification and Increased Cumulative Dose of Postoperative Adriamycin-Cyclophosphamide (AC) Therapy With G-CSF on the Disease-Free Survival and Survival of Patients With Primary Breast Cancer and Positive Axillary Nodes
Specific Aims
- To determine whether giving larger but fewer doses of CY (dose intensification) in an AC combination will more effectively prolong disease-free survival and survival than does the same cumulative dose of CY given over a more prolonged period of time.
- To determine whether increasing the dose intensity as well as the cumulative dose of CY (longer administration of a higher dose) will result in greater prolongation of disease-free survival and survival than does giving CY at the same dose intensity but for a shorter period of time (lower cumulative dose).