NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-29
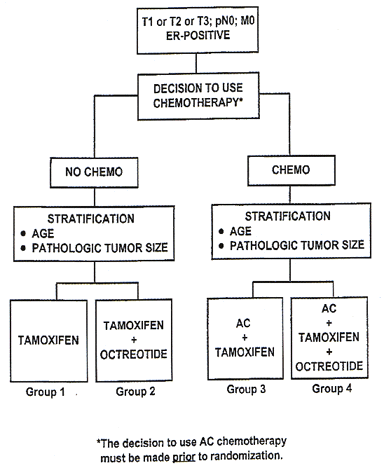
A Clinical Trial to Evaluate the Benefit of Adding Octreotide (SMS 201-995 PA LAR) to Tamoxifen Alone or Tamoxifen and Chemotherapy in Patients with Axillary Node-Negative, Estrogen-Receptor-Positive, Primary Invasive Breast Cancer
Specific Aims
The primary aim of this study is to determine if the addition of octreotide (SMS 201-995 pa LAR) to tamoxifen alone or to tamoxifen in combination with chemotherapy prolongs disease-free survival (DFS) in women with axillary node-negative, estrogen-receptor-(ER)-positive breast cancer. Secondary aims include determining if the addition of octreotide prolongs survival, and if the rates of endometrial cancer and opposite breast cancer are altered by the concomitant administration of octreotide and tamoxifen. An additional secondary aim of this study is to determine accurate rates of several adverse events that have been reported in patients taking octreotide.