NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
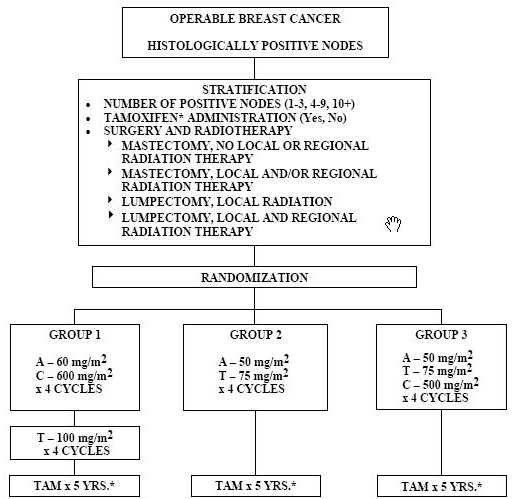
Protocol B-30
A Three-Arm Randomized Trial To Compare Adjuvant Adriamycin And Cyclophosphamide Followed By Taxotere (AC6T); Adriamycin And Taxotere (AT); And Adriamycin, Taxotere, And Cyclophosphamide (ATC) In Breast Cancer Patients With Positive Axillary Lymph Nodes
Specific Aims
The primary aims of this phase III trial are to determine whether four cycles of post-operative Adriamycin (A: 50 mg/m2) and Taxotere (T: 75 mg/m2) and cyclophosphamide (C: 500 mg/m2) will more effectively prolong disease-free survival (DFS) and overall survival (S) of patients with node-positive breast cancer than will four cycles of AC (A: 60 mg/m2 and C: 600 mg/m2) followed by four cycles of Taxotere (100 mg/m2), and if four cycles of post-operative AT (A: 50 mg/m2 and T: 75 mg/m2) is at least as efficacious as the regimens containing cyclophosphamide. Secondary aims include comparing toxicities among the three regimens; comparing the quality of life (QOL) among breast cancer patients receiving one of the following treatments: ATC, AC→T, or AT; and examining differences in amenorrhea in premenopausal women in each treatment arm, and its relationship to symptoms, QOL, and DFS and S.