NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-31
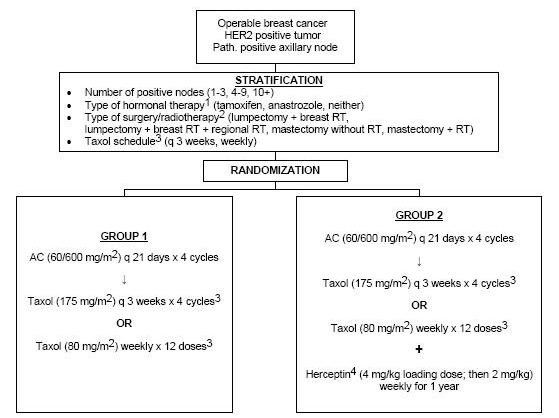
A Randomized Trial Comparing The Safety And Efficacy Of Adriamycin And Cyclophosphamide Followed By Taxol (AC6T) To That Of Adriamycin And Cyclophosphamide Followed By Taxol Plus Herceptin (AC6T + H) In Node-Positive Breast Cancer Patients Who Have Tumors That Overexpress HER2
Specific Aims
The primary aim of stage 1 of this trial is to compare the cardiotoxicity of four cycles of Adriamycin and cyclophosphamide (AC) followed by four cycles of Taxol, with that of the same chemotherapy regimen plus Herceptin, in patients with operable, histologically node-positive breast cancer which overexpresses the HER2 protein. The primary aim of stage 2 of this trial is to determine whether four cycles of AC followed by four cycles of Taxol and weekly Herceptin for 1 year, with or without tamoxifen for 5 years, is more effective in prolonging Survival than four cycles of AC followed by four cycles of Taxol, with or without tamoxifen, in patients with operable, histologically node-positive breast cancer which overexpresses the HER2 protein. A secondary aim of the trial is to determine whether four cycles of AC followed by four cycles of Taxol and weekly Herceptin for 1 year, with or without tamoxifen for 5 years, is more effective in prolonging DFS than four cycles of AC followed by four cycles of Taxol, with or without tamoxifen, in patients with operable, histologically node-positive breast cancer which overexpresses the HER2 protein.