NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol B-34
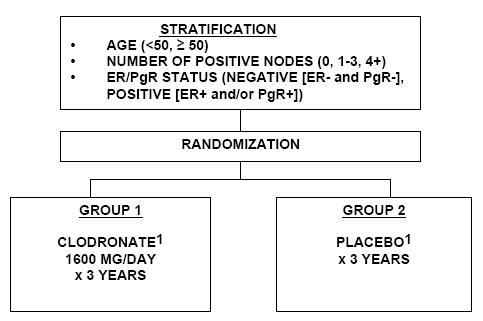
A Clinical Trial Comparing Adjuvant Clodronate Therapy vs. Placebo in Early-Stage Breast Cancer Patients Receiving Systemic Chemotherapy and/or Tamoxifen or No Therapy
Specific Aims
The primary aim of this phase III trial is to determine whether clodronate administered for 3 years either alone or in addition to adjuvant chemotherapy and/or hormonal therapy, in patients with early-stage breast cancer will improve disease-free survival. Secondary aims include determining whether clodronate will reduce the incidence of skeletal metastases, improve overall survival, improve relapse-free survival, reduce the incidence of non-skeletal metastases, and reduce the incidence of skeletal morbidity (skeletal fractures, hypercalcemia, skeletal pain, need for radiation therapy, spinal cord compression). Also to investigate the relevance of serum markers of bone turnover as a prognostic factor for the development of bone metastasis.