NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
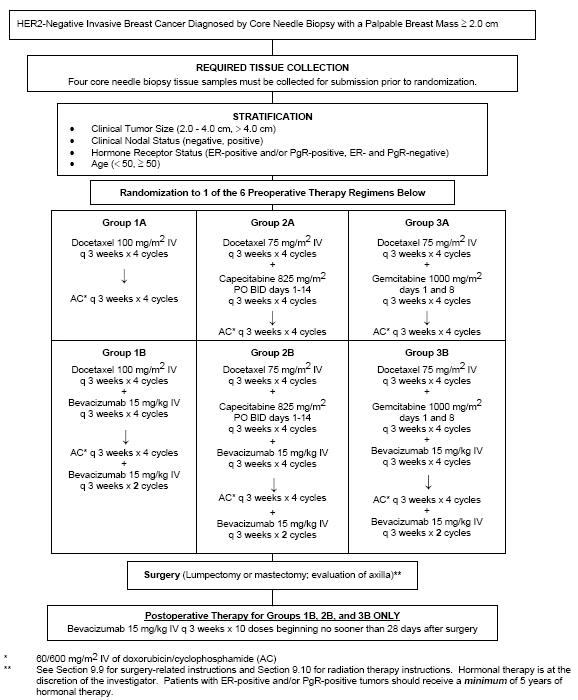
Protocol B-40
A Randomized Phase III Trial of Neoadjuvant Therapy in Patients with Palpable and Operable Breast Cancer Evaluating the Effect on Pathologic Complete Response (pCR) of Adding Capecitabine or Gemcitabine to Docetaxel when Administered Before AC with or without Bevacizumab and Correlative Science Studies Attempting to Identify Predictors of High Likelihood for pCR with Each of the Regimens
Specific Aims
The primary aims of this Phase III study are to determine whether adding capecitabine or gemcitabine to docetaxel followed by AC will increase the pathologic complete response (pCR) rates in patients with palpable and operable HER2-negative breast cancer and to determine whether the addition of bevacizumab to three docetaxel-based regimens followed by AC will increase pCR rates.