NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
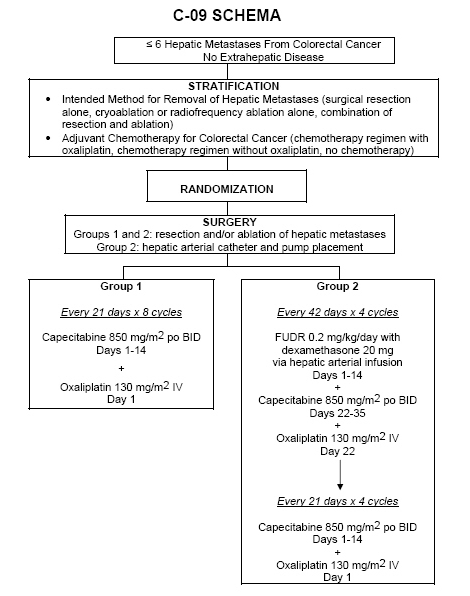
Protocol C-09
A Phase III Clinical Trial Comparing Oxaliplatin, Capecitabine and Hepatic Arterial Infusion of Floxuridine to Oxaliplatin and Capecitabine in Patients with Resected or Ablated Liver Metastases from Colorectal Cancer
Specific Aims
Primary Aim
The primary aim of this study is to compare progression-free interval (PFI) between the two treatment groups.
Secondary Aim
The secondary aims of this study are to:
- compare overall survival and liver PFI between the two treatment groups;
- assess toxicity in each of the treatment regimens;
- compare self-reported symptoms between two treatment groups; and
- compare quality of life in each of the treatment regimens.