NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
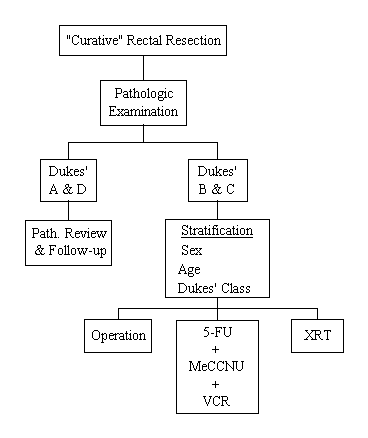
Protocol R-01
A Clinical Trial to Evaluate Postoperative Radiation and Postoperative Systemic Chemotherapy in the Management of Resectable Rectal Carcinoma
Specific Aims
To determine if the disease-free survival and survival of patients having a "curative" resection for rectal carcinoma is improved by (1) the addition of postoperative radiation therapy or (2) the addition of the three-drug regimen, 5-FU, Methyl-CCNU, and vincristine (MOF). This study should also provide information relative to the pathology of rectal cancer that will contribute to greater understanding of the natural history of this disease so that subsets of patients who are at higher risk of recurrence can be identified.