NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
Protocol R-02
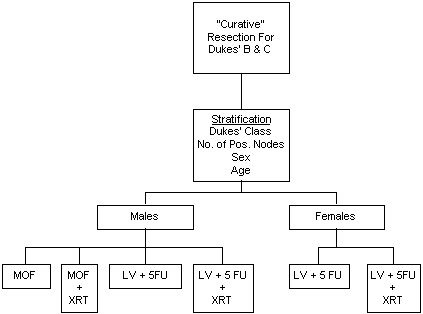
A Protocol to Compare Adjuvant MeCCNU, Vincristine and 5-Fluorouracil (MOF), With and Without Radiation, to Adjuvant Leucovorin and 5-Fluorouracil (LV+5FU), With and Without Radiation, in Patients with Dukes' B and C Carcinoma of the Rectum
Specific Aims
Protocol R-02 has been designed to determine if the addition of radiation therapy to chemotherapy improves disease-free survival and survival in patients with Dukes' B and C carcinoma of the rectum. Based on findings in Protocol R-01, males are randomized to one of four treatment regimens: MOF, MOF+RT, LV+5FU, or LV+5FU+RT; females are randomized to one of two treatment regimens: LV+5FU or LV+5FU+RT. Specific aims are: (1) to determine whether radiation, when added to a chemotherapeutic regimen, prolongs disease-free survival and survival; (2) for males only, to compare MOF, with and without radiation, to LV+5FU, with and without radiation; and (3) for males and females, to compare LV+5FU without radiation to LV+5FU with radiation.