NSABP Members' Area
Password Protected - Access
Limited to NSABP Participating
Institutions Only
NSABP Foundation, Inc.
General NSABP Information
Financial Conflicts of
Interest Policy
Contact the NSABP
Employment

Clinical Trials Information
Clinical Trials Overview
Protocol Chart
Never Say Lost
Treatment Trials Information
Protocol B-51
Protocol B-52
Protocol B-53/S1207
Protocol B-55/BIG 6-13
Prevention Trials Information
Protocol P-1 - BCPT
Protocol P-2 - STAR
To report problems, ask
questions or make comments,
please send e-mail to:
Webmaster@nsabp.pitt.edu
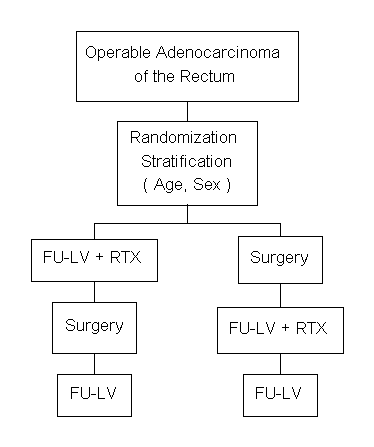
Protocol R-03
A Clinical Trial to Evaluate the Worth of Preoperative Multimodality Therapy (5FU+LV+RTX) in Patients with Operable Carcinoma of the Rectum
Specific Aims
The primary purpose of this study is to determine whether the administration of preoperative chemotherapy (5FU+LV) and radiotherapy, followed by postoperative chemotherapy, is more effective than the administration of postoperative chemotherapy and radiotherapy in improving the disease-free survival and survival of patients with operable carcinoma of the rectum. Secondary aims of the study include the assessment of the effect of preoperative therapy on tumor response, tumor downstaging, rate of local recurrence, and type of operation (sphincter-saving procedures versus abdominoperineal resections).